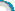一、有关化学反应和溶液的综合计算
1.用质量分数为5%的稀盐酸和10%的氢氧化钠溶液进行中和反应实验时,反应过程中溶液的酸碱度变化如图1所示:
(1)该实验是将__ __(选填“稀盐酸”或“氢氧化钠溶液”)溶液滴加到另一种溶液中。
(2)当加入溶液的质量为20 g时,求所得溶液中溶质的质量。
图1
2.[2014•永州]“氯碱工业”是我国目前化学工业的重要支柱之一,它的主要原理是电解饱和食盐水,其化学方程式为xNaCl+2H2O=====通电xNaOH+H2↑+Cl2↑。在20 ℃时,取100 g饱和NaCl溶液进行电解,一段时间后测得产生氯气(Cl2)的质量为7.1 g。已知:20 ℃时,NaCl的溶解度为36 g。
请分析并计算回答:
(1)运用质量守恒定律可知上述反应中x=__ __。
(2)通过计算,上述过程中同时产生H2的质量为_ __g。
(3)计算电解后剩余溶液中NaCl的质量分数(写出详细的计算过程,结果精确到0.1%)。
|